What are Minerals?
What are Minerals?
Minerals are the building blocks of rocks. In order to be considered a mineral, it must fit specific definitions including:
Minerals are the building blocks of rocks.
Naturally Occurring
This means they are formed in nature. Humans have developed technology to make minerals synthetically in a laboratory. These man-made “minerals” are not actually minerals. For example, cubic zirconium is not a mineral because it is only made in a laboratory. Halite and quartz are both minerals that can be made in a laboratory but also found in nature.
Minerals must be solid in a natural state. In some instances, liquid or gases may eventually form into minerals, but they are not considered minerals until they are solid. The one exception to this rule is native mercury which crystallizes only at -39 degrees Celsius. This is because the International Mineralogical Association included it as mineral before the current rules were established.
Composed of elements
Minerals, like all matter, are made out of chemical elements. Elements are substances that are made up of only one kind of atom (remember the periodic table?). Elements have specific chemical and physical properties and cannot be broken down into other substances. Some minerals are made of only one type of element, for example gold, while other minerals are made of a combination of elements, such as quartz which is a combination of silicon and oxygen. Minerals are classified by their types based on their chemical composition and include:
- Silicates: the largest group of minerals, made of Silicon and Oxygen (SiO2). Quartz is a type of silicate.
- Native Elements: elements that have a distinct crystalline structure but are not combined with any other elements. These include copper, gold, silver, etc.
- Sulfides: minerals made with sulphur (S2) combined with other elements (excluding oxygen). These minerals are commonly found with metal ores and include galena and pyrite.
- Sulfates: minerals made with Sulphur and Oxygen (SO4). These minerals are commonly found in evaporate conditions (deserts) and include gypsum, common in building materials.
- Oxides: minerals with at least one oxygen atom combined with another atom (not oxygen). Oxides are most common in the Earth’s crust and are typical in metal-bearing minerals (magnetite and hematite).
- Carbonates: minerals made with carbon and oxygen (CO3). Carbonates are inorganic, but can be found within living organisms. These minerals are the main components of carbonate rocks, such as limestone and dolomite.
- Halides: minerals with a dominant halide element (fluorine, chlorine, bromine, and iodine – F, Cl, Br, and I).
Crystalline Structure
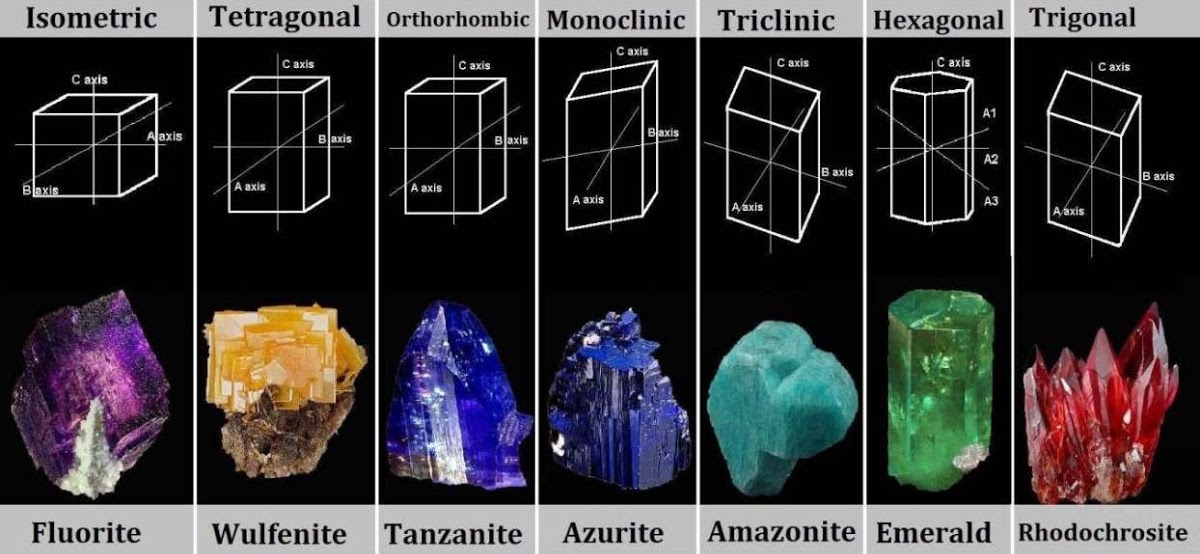
A crystalline structure is an ordered, repeated way in which atoms are arranged. This allows a mineral to grow in specific patterns. Crystal structure is the reason minerals will show specific physical properties such as cleavage (how minerals break) and symmetry, making them easier to be identified. Although minerals may differ in other ways, there are only 7 crystal families that a mineral can form: isometric, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and trigonal.
Some minerals are considered polymorphs, which means they may have the same chemical composition as another mineral, but form in a completely different way. A good example of this is carbon and diamonds. Both of these minerals are made from carbon, but they form in very different ways. Graphite, the “lead” used in pencils, is very soft and breaks easily, whereas diamonds are the hardest natural mineral. This is a result of the carbon atoms bonding or connecting to each other in different ways.
Inorganic
In chemistry, organic refers to chemical compositions, or compounds, that contain carbon that is bonded to hydrogen and found in living things. This includes proteins, carbohydrates (sugars), lipids (fats), and nucleic acids (DNA and RNA). These are complex molecules and are found exclusively in living organisms. These compounds are typically quite large and complex.
Inorganic compounds are typically smaller molecules with a much simpler chemical structure. A general rule is that inorganic compounds do not contain carbons. However, there are some carbon-based minerals that are inorganic including carbides, carbonates, and cyanides.





